Thermal Energy
The total amount of kinetic and chemical potential energy possessed by the particles of a substance. (Kinetic - motion of the particles; Potential - force of attraction between the particles) It is not the same as Heat
It Depends on
- How fast the particles of a substance move
- The quantity of particles in a substance
Kinetic Molecular Theory
Matter is composed of particles (atoms and molecules) that attract each other and have kinetic energy.
Since these particles have kinetic energy, they are in a state of constant motion
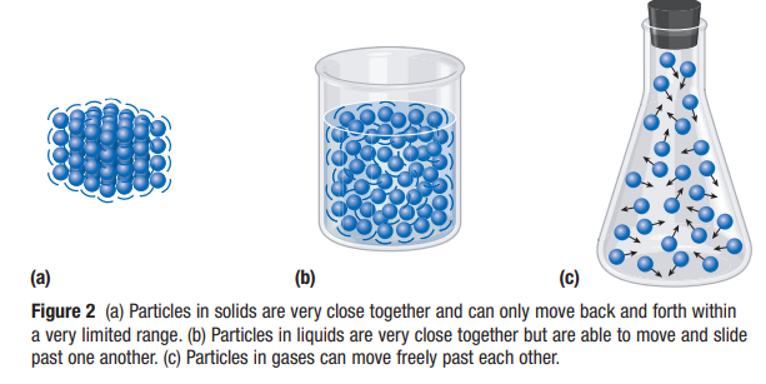
| State | Kinetic Energy | Particle Movement |
|---|---|---|
| Solid | Very Little | Vibrate only |
| Liquid | More than solids | Move and slide past one another (flow) |
| Gas | A lot | Move freely and rapidly past each other |
What is Temperature?
The average Kinetic Energy of the particles in an object
Why can’t we measure Thermal Energy?
We cannot measure the total amount of thermal energy in an object because it is impossible to measure the kinetic and potential energy of every particle within the object.