Changing States
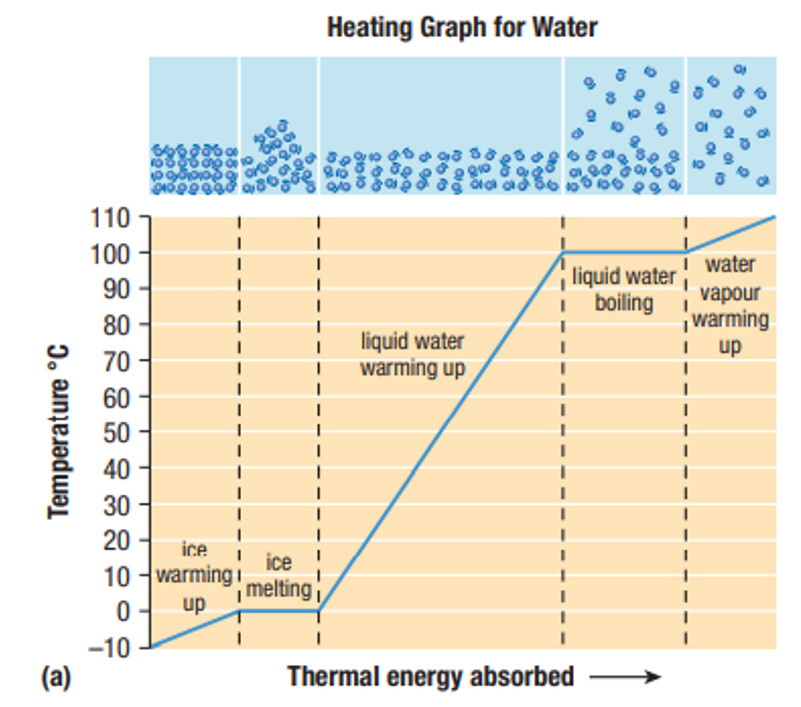
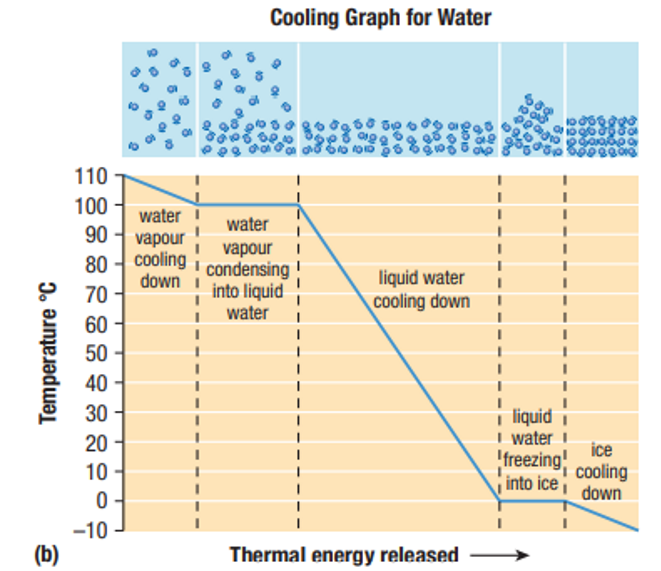
Why does the temperature not change during phase changes?
Thermal Energy is being transformed into Chemical Potential Energy to make or break bonds, not Kinetic Energy, therefore, the temperature does not change.
- Melting and boiling: Thermal Energy absorbed by the particles of a substance is converted to Chemical Potential Energy to break the bonds that hold the particles together
- Condensation and freezing: Thermal Energy is released to allow the particles to move closer together and become more organized