the mathematical description of a chemical system at equilibrium
Example:
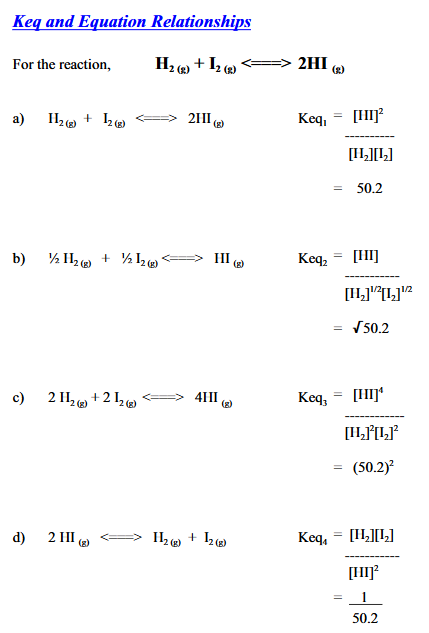
- The coefficients from the balanced chemical equation become the exponents of the concentrations in the equilibrium law.
Using it in practice
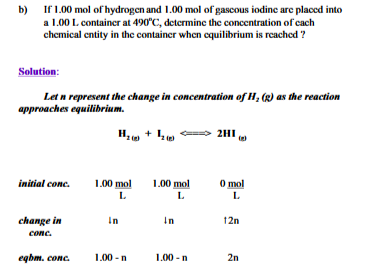
The following equilibrium concentrations were obtained at 490 degrees
IMPORTANT
Only equilibrium concentrations can be substituted into equilibrium law
which means that there is 45.9x more product than reactant at this temperature. The value of changes depending on the temperature, but is constant when changing concentrations.
IMPORTANT
In this example, there is no unit because the mol/L cancel out. BUT there will never be one unit for the equilibrium constant.
Since Then
Therefore
and
Significance of different values
If is very large, the equilibrium must lie far to the right since product concentrations must be greater than reactant concentrations for the numerator to be greater than the denominator in the equilibrium law.
If is very small, the equilibrium must lie far to the left since reactant concentrations must be greater than the product concentrations for the denominator to be greater than the numerator in the equilibrium law.
When = 1, the equilibrium does not lie in any particular direction because the reactant concentrations must be very close to the product concentrations.
Temperature Effects of the Equilibrium Constant
- For the reaction Increasing the temperature causes the equilibrium to shift to the right as predicted by Le Chatelier’s Principle. This increases the concentration of the products . Therefore for endothermic reactions, increases as temperature increases and decreases if temperature decreases
- For the reaction Increasing the temperature causes the equilibrium to shift to the left. This increases the concentration for the reactant . Therefore, for exothermic reactions, increases as the temperature decreases and decreases if the temperature increases.
| Type of Reaction | Temperature Change | change |
|---|---|---|
| endothermic | increases | increases |
| endothermic | decreases | decreases |
| exothermic | increases | decreases |
| exothermic | decreases | increases |
Solids and Liquids in equilibrium?
Heterogenous Systems: one which involves a reaction between 2 or states of matter. Homogenous Systems: a reaction where all participants are present in the same state.
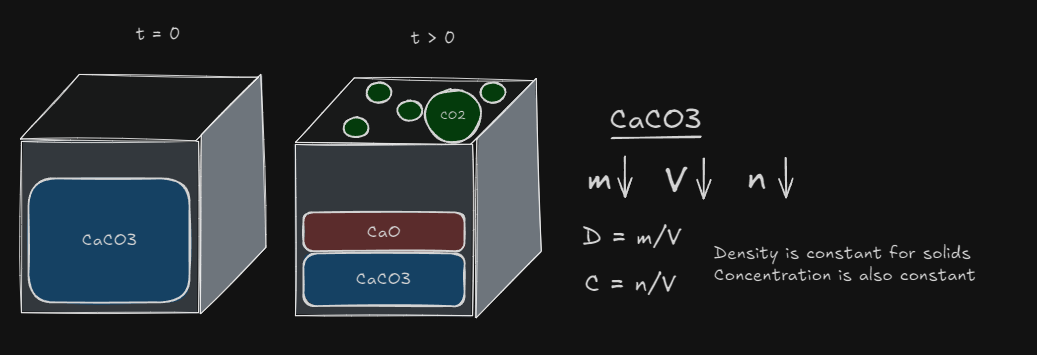
The concentration of pure solids or pure liquids cannot change.
Title
Whenever dealing with heterogenous systems, always remove the solid and liquid concentrations Concentration only work for aqueous and gases substances