When all of the solute in a solution is fully dissolved (fully saturated), the solution is in a state of heterogenous equilibrium.
In this example, is the solid, and therefore has a constant concentration because this is a heterogenous equilibrium. It is assumed that is on the left side of the equation because the products are aqueous Solubility Product Constant: This is the product of the solubilities of each ion in the solution.
- If it is very large Equilibrium lies far to the right and prefers the forward reaction
- If it is very small Equilibrium lies far to the left and prefers the reverse reaction.
Molar Solubility for Salts
The amount of solute (salt) dissolved in 1.0L of its saturated solution
NOTE
Salts are any ionic compound that doesn’t contain or
Ex. and
Not Bases. and Not Acids. and Or Oxides. and
Let x represent the molar solubility of lead(II) iodide.
| ↓x broken apart | ↑x created | ↑2x created |
therefore,
Therefore the molar solubility of plumbous iodide is 1.3 x 10⁻³ mol/L.
Common Ion Effect
The phenomenon that occurs when a salt tries to dissolve into a solution that has one or both of the ions in the salt.
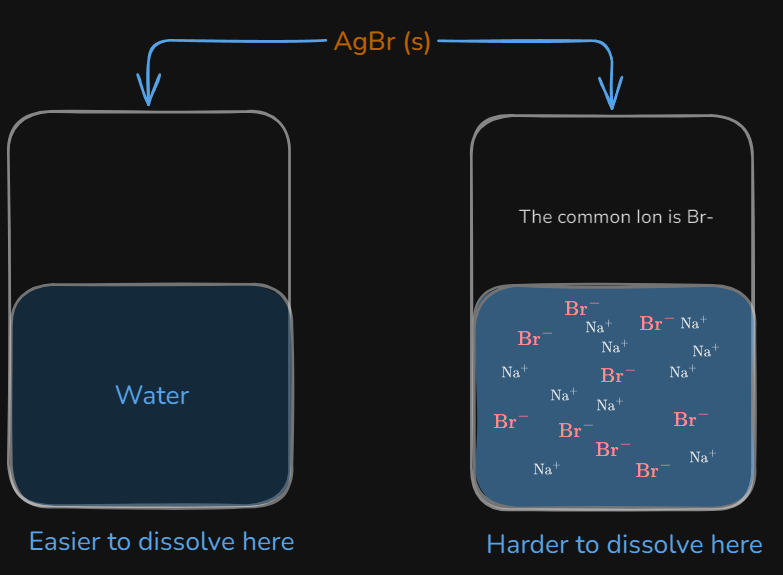 The is greater compared to an aqueous environment (pure water). This makes it harder for to dissolve into it
The is greater compared to an aqueous environment (pure water). This makes it harder for to dissolve into it
Steps to Solve Common Ion Problems
- Find Molar Solubility in Water
- Find final concentration of solution with common ion
- Find molar solubility in the solution with the common ion
Compare the molar solubility of in pure water to a 0.10 mol/L solution of .
of at 25°C is .
 Let x represent the molar solubility of in water.
Let x represent the molar solubility of in water.
| Initial | 1x | 0 | 0 |
| Final | 0 | 1x | 1x |
Therefore, the molar solubility in water is .
Recall:
| Initial | 0.10 mol/L | 0 mol/L | 0 mol/L |
| Final | 0 mol/L | 0.10 mol/L | 0.10 mol/L |
- Since is extremely soluble in water according to solubility rules (grade 11), it is expected that this solid completely dissociates into ions. There is no equilibrium. here!
When trying to dissolve silver bromide in instead of water, let x represent the molar solubility of silver bromide.
Very Important
We are going to assume that is a very small number to where so we can assume that is 0
Therefore, the molar solubility in water is where in is