Radioactive Decay
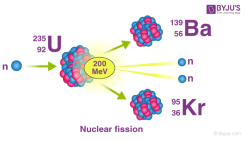
When unstable isotopes try to balance themselves by getting rid of their excess protons or neutrons
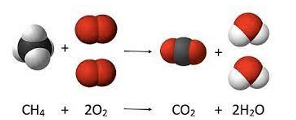
Chemical vs Nuclear Reactions
Chemical
- The interaction of substances to form new substances
- The identities of the elements do not change; only their organization changes
Nuclear
- Involve changes in the nucleus of an atom, sometimes resulting in new elements
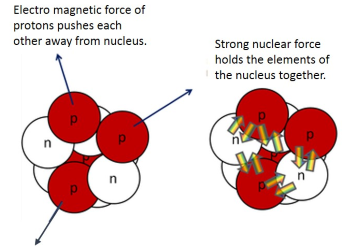
- The nucleus of an atom contains protons (+) and neutrons (0)
- The positively-charged protons repel each other (electrostatic forces)
- However, the nucleus is held together by the strong nuclear force which is always attractive and much stronger than electrostatic forces
Unstable Isotopes
- Atoms with too many protons or too many neutrons, upsetting the strong nuclear forces
- Try to balance themselves by getting rid of their excess protons or neutrons
- Unstable nuclei are radioactive and emit Radiation
Radiation
The emission of Energy in the form of waves or fast-moving particles
The higher the frequency of an electromagnetic-wave, the more energy it carries
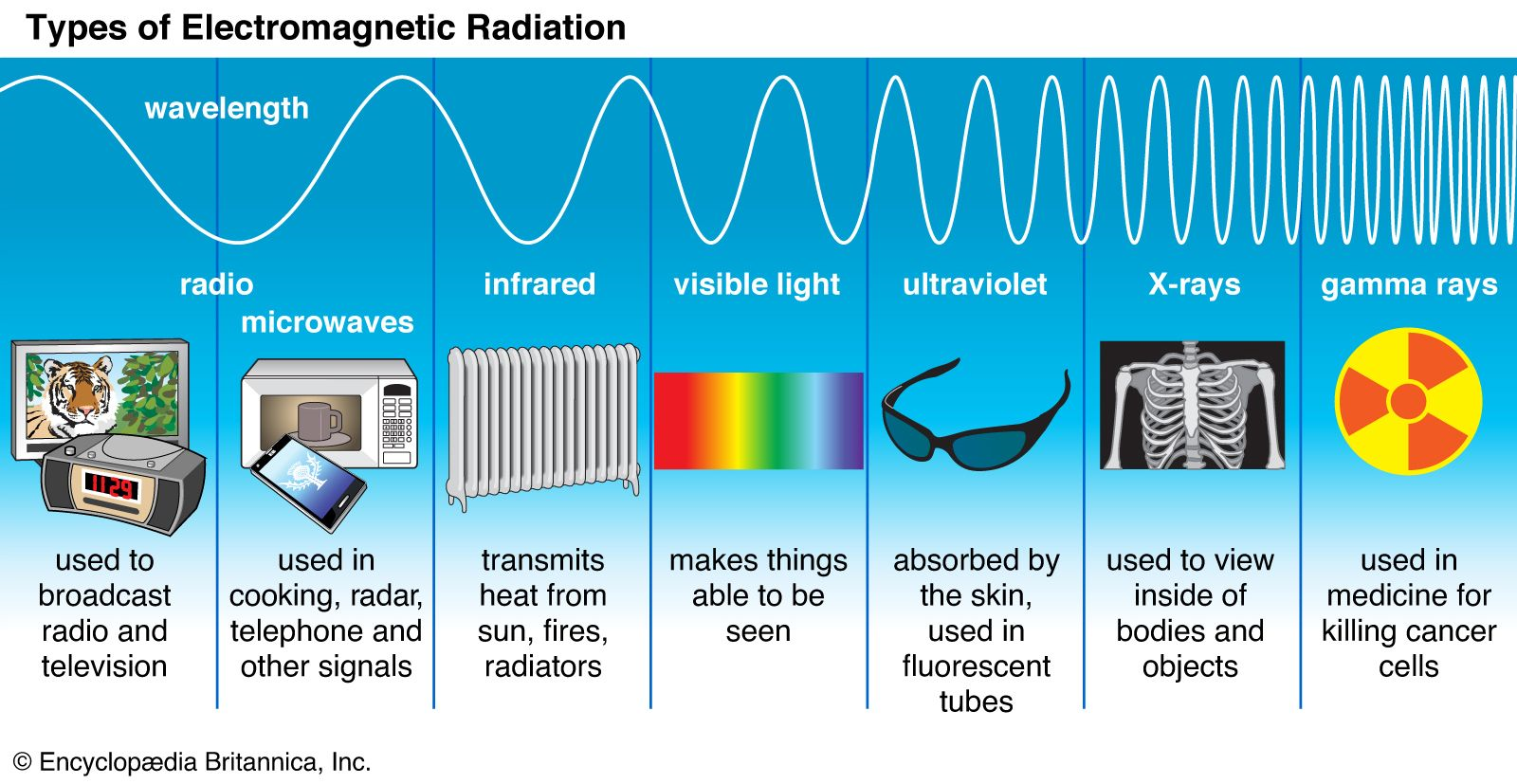
Ionizing Radiation (Gamma, X-Ray)
Waves or particles that carry enough energy to remove an electron from an atom or molecule
Types of Radioactive decay
Alpha Decay
It is the most common form of decay. A helium nucleus (two protons and two neutrons) is spontaneously emitted from the nucleus
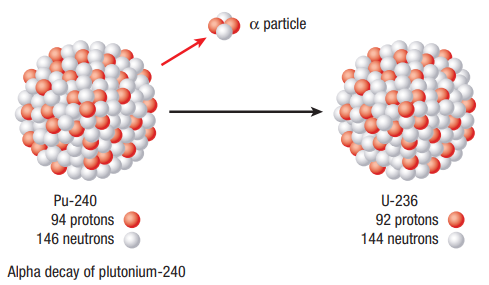
Beta Declay
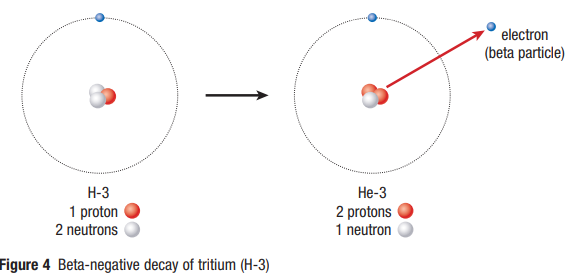
Beta-Negative Decay
- When a nucleus contains too many neutrons, the strong nuclear force becomes much greater than the electrostatic force - A neutron will spontaneously decay into a proton and electron to maintain stability
- An electron is emitted from the nucleus of a parent atom
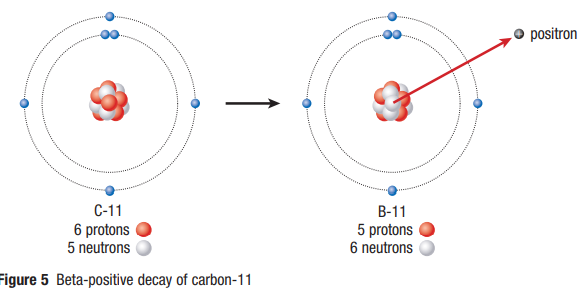
Beta-Positive Decay
- A proton changes into a neutron and a positron (a particle with a positive charge and the same mass as an electron
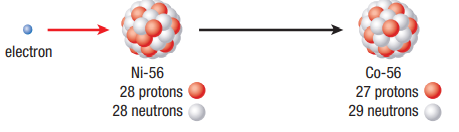
Electron Capture
An electron is absorbed by a nucleus and combines with a proton to form a neutron Mass stays the same but a proton is lost
Gamma Decay
- After a nuclear reaction has occurred, the daughter (new) nucleus is an a high-energy (excited) state
- The nucleus spontaneously releases energy (gamma ray) to return to a lower, more stable energy state
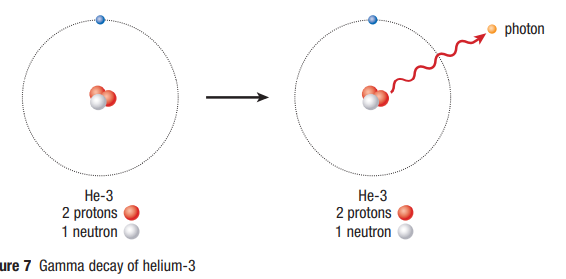
Gamma ray
highly energetic form of electromagnetic radiation emitted as a photon (high energy particle with no mass)