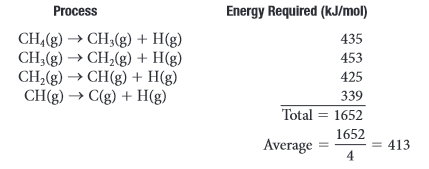
Bond Dissociation Energy: the quantity of energy required to break a chemical bond. Measured in
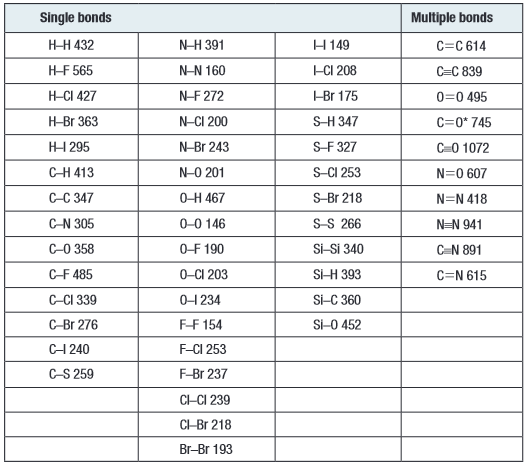
Info
They are measured in averages because the bond energy of a bond is affected by the number of atoms and bonds around it. Using an an average is more convient
Trends in Bond Energies
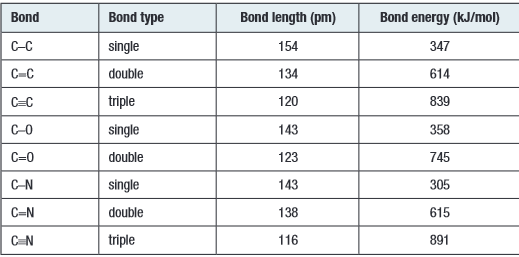
- Multiple bonds are generally stronger than single bonds
- As the number of bonds increases, the bond length shortens
What happens in a reaction?
- The bonds in the reactants break first. For bonds to be broken, energy must be added (endothermic).
- Then new bonds are made in the products which releases energy (exothermic)
sum of energies required to break old bonds - sum of energies released in the formation of new bonds

energy required is the reactants bond energies and energy released is the products bond energies is the amount in moles of each reactant or product
using Bond energies