If the overall equation for a reaction can be expressed as the algebraic sum of the equations of two or more other reactions, then the of that reaction is the algebraic sum of the for those reactions
Example
Nitrogen Dioxide can be created in one step
Or in two steps
If you add the two equations
Then you’ll get the one step equation at the top
Energy State Diagram
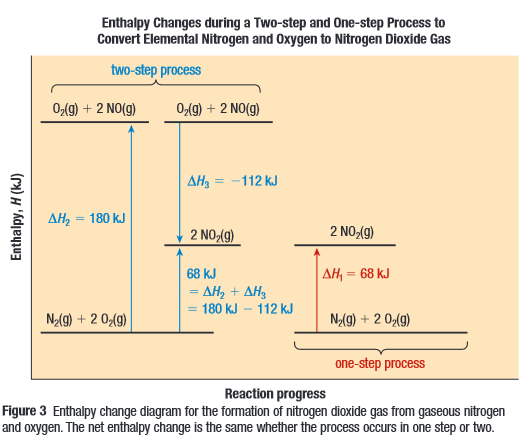
Rules of Enthalpy Changes
- If you reverse a chemical reaction, you must also reverse the sign for
- If the moles of every reactant and product is multiplied, then is multiplied by that same number